A Computational Approach to Precision Oncology for TP53 Mutations
- Srijon Mandal
- Jul 25, 2025
- 2 min read
TP53 is a critical tumor-suppressor gene, plays a pivotal role in preventing cancer. Mutations in TP53 are linked to over 50% of human cancers, making it crucial for precision oncology. This research uses advanced computational techniques and machine learning models to analyze TP53 mutations, providing insights into their impact and aiding in the development of targeted therapies.

Introduction
Recently I have done extensive research to understand how to use statistical and machine learning algorithms for analyzing TP53 mutations across various cancer types in order to enhance diagnosis, prognosis, and therapy development. This research approach aims to improve patient outcomes and advance personalized medicine.
Methodology
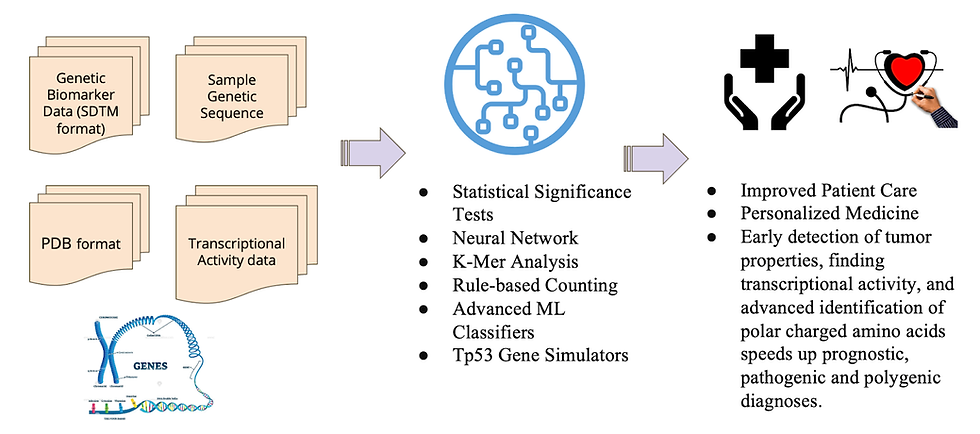
I developed a unified framework which combines multiple computational approaches:
Statistical Modeling and Significance Tests: Evaluates genetic sequences using one-sided T-tests and p-values to determine mutation likelihood.
Machine Learning (ML) Techniques: Utilizes K-Mer analysis, Random Forest classifiers, and neural networks to identify specific mutations and predict tumor types and properties.
Transcriptional Activity Prediction: Incorporates biophysical simulation data and PCA (Principal Component Analysis) to forecast transcriptional behavior of mutant p53 proteins.
Key Findings
The customized ML models demonstrated ~97% accuracy in predicting whether TP53 mutations result in malignant or benign tumors.
Statistical significance tests identified overexpression patterns linked to cancer metastasis, offering new pathways for early intervention.
Rule-based counting techniques detected polar-charged amino acid variations, highlighting mutations affecting the protein's electrostatic potential.
Survival analysis using Kaplan-Meier models provided insights into TP53 mutations' effects on patient outcomes.
Conclusion
My comprehensive computational strategy offers healthcare professionals a powerful tool for making informed decisions about TP53 mutations. By integrating various statistical and ML techniques, the research paves the way for AI-driven precision oncology, enhancing diagnosis, treatment, and prognosis for cancer patients.




Comments